
What Cruelty-Free Labels Truly Mean
By Caiden Feldman | January 15th, 2026
"Without regulatory standards, cruelty-free labeling reflects marketing strategies rather than scientific standards.”
Image by alvaro_cabrera

Xenotransplantation: Clinical Progress and a Need for Regulation
By CriShaun Hardy | December 18th, 2025
"As xenotransplantation moves forward in formal clinical trials, greater consideration must be given to how future regulation and ethics will shape the evaluation of its feasibility and long-term integration into the organ transplantation framework.”

AOPs, Space, and In-Silico
By Awurakua Asamoah-Mensah | October 16th, 2025
"AOP development is pivotal in guiding regulatory decisions, bridging knowledge gaps, and predicting stimulus impact. Further integrating new approach methodologies, such as in-silico models, into research will enhance AOP development, offering novel and relevant contributions to science, medicine, and policy."
Image by WikiImages
Transitioning to Human-Centered Science: An Off-Ramp and Transition Plan
By Paul Locke | September 4th, 2025
“For those of us who have been advocating for the development, use and adoption of human centered NAMs, the Roadmap and these announcements are a breath of fresh air. It seems that – finally – the federal government has turned the corner. But talking the talk is not enough. The transition to human centered science and away from animal testing needs an implementation plan …”
Image by FDA
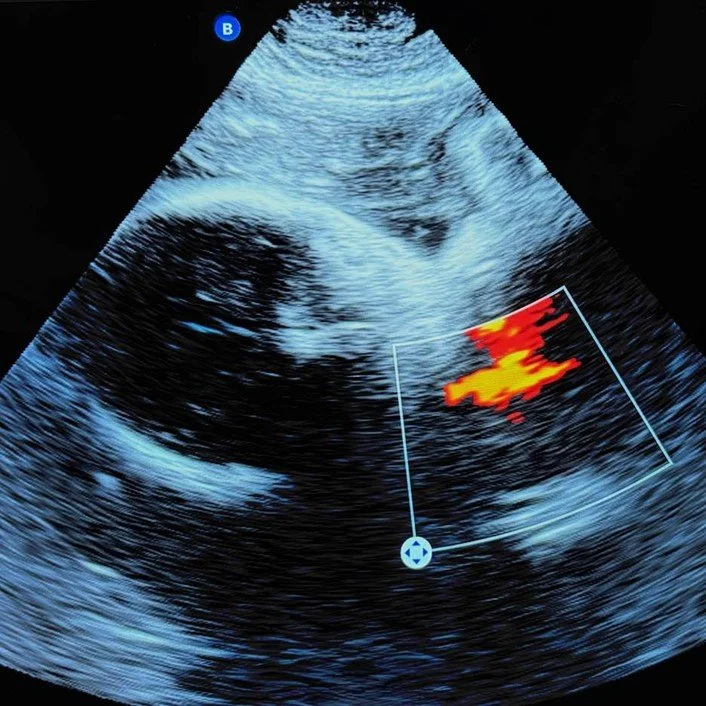
A Bright Tomorrow for Therapeutic Orphans: The Potential for New Approach Methodologies to Bridge Data Gaps for Pregnant Patients
By Matthew Durthaler | July 24th, 2025
“As caution often precludes pregnant humans from serving as models of their own physiology, various non-human animal models have been employed. While these models have displayed some value, they are severely limited by interspecies variation and thus serve as subpar predictors of gravid human physiology.”
Sonogram captured by Matthew Durthaler, used with permission

Our Tiny Allies in Toxicological Testing Part 2 – Transparent Roundworms as Alternative Models
By Aaradhya Diwan | July 10th, 2025
“There are numerous opportunities for using C. elegans as a model for toxicity testing, thanks to their unique advantages and translational relevance to human health, as demonstrated in applications to neuroscience and developmental biology.”
Image by Zeynep F. Altun

From Roadmap to Reality: Validating NAMs for FDA's Plan to Phase Out Animal Testing
By Yiguang Zhu | May 15th, 2025
“The FDA and the broader scientific community recognize NAMs as a means to obtain 'faster and more accurate human risk assessments' while reducing animal use.”
Image by ChatGPT

Organoid’s Potential to Advance Low Dose Research
By Loza Taye | April 3rd, 2025
“Organoids will have an increasingly important role in shaping our understanding of radiation biology and in the development of policies and medical practices concerning radiation exposure.”
Image by aislan13
Advancing non-animal science and public health progress in the 119th Congress – going for the win-win
By Dr. Paul Locke | March 6th, 2025
“[R]ather than looking back at past accomplishments, it is better to explore what we need for future success in public health and medicine.”
Image by Mike Gillis

Collaboration Over Competition: Advancing the Use of Microphysiological Systems
By Dr. Anicca Harriot | February 20th, 2025
“Federal agencies have the power to define a clear path that drives MPS forward in to further reducing the use of animal testing.”

Professional Perspectives: Refinement in Animal Research: A Crucial Step Toward Ethical Progress
By Sally Thompson-Iritani | February 6th, 2025
“Incorporating refinements in animal research is not optional—it is essential in modern science. The rapid pace of technological advancements and increased public scrutiny make it imperative to adopt comprehensive refinement strategies.“

Unleashing Innovation: The Business Case for New Approach Methods (NAMs)
By Zachary Liebowitz | January 23rd, 2025
“As we stand on the cusp of a new era in science and industry, the case for NAMs is clear. They are more than a set of tools—they represent a paradigm shift that aligns innovation with ethical responsibility and economic growth.“

The Utility of an Intra-Agency NAM Office
By Breanne Kincaid | January 10th, 2025
“…the FDA’s NAM office represents a strategic evolution in integrating innovative methodologies into regulatory science. While it complements ICCVAM’s mission, its intra-agency focus and regulatory authority position it to drive faster, more context-specific advancements in risk assessment.“

Our Tiny Allies in Toxicological Testing - Zebrafish and their Embryos as Alternative Models
By Aaradhya Diwan | December 19th, 2024
“[Zebrafish] models offer powerful insight for detecting the negative impacts of chemical substances on ecosystem health and human health.”

ICCVAM 2024 Public Forum Highlights
By Loza Taye | June 13th, 2024
“The 2024 ICCVAM Public Forum highlighted progress and future directions in alternative testing methods and how that work is proceeding in federal agencies and some partners. The presentations and discussions underscored a shared commitment to reducing animal testing while improving regulatory science and maintaining rigorous safety standards.”

Innovative Solutions for an Invisible Threat: Enhancing PFAS Assessment
By Loza Taye | May 2nd, 2024
“The PFAS public health threat demands rapid and sustained action and innovative solutions. Increasing evidence indicates that NAMs should be prioritized as a path to tackle the sizeable chemical class with speed and efficacy.”