
Not Just Test Subjects: Remembering Laboratory Animals
By Daria Bednarczyk | January 29th, 2026
“Choosing to remember laboratory animals does not reject science; it reminds us to pursue research with greater humanity, grounding discovery and innovation in the millions of animal lives sacrificed in the name of progress.”

What Cruelty-Free Labels Truly Mean
By Caiden Feldman | January 15th, 2026
"Without regulatory standards, cruelty-free labeling reflects marketing strategies rather than scientific standards.”
Image by alvaro_cabrera

Xenotransplantation: Clinical Progress and a Need for Regulation
By CriShaun Hardy | December 18th, 2025
"As xenotransplantation moves forward in formal clinical trials, greater consideration must be given to how future regulation and ethics will shape the evaluation of its feasibility and long-term integration into the organ transplantation framework.”

Environmental Health and Safety (EHS) in the Animal Industry
By Richelle Romanchik | December 4th, 2025
"As regulations and industry standards continue to evolve, we must not lose sight of the people who care for these animals and carry out the daily work of in vivo toxicological studies... If we fail to adequately protect the workers in these facilities, how can we expect them to protect the animals entrusted to their care?.”

Reflections on “From Deference to Deliberation”
By Loza Taye | November 13th, 2025
"...it's still too soon to know the exact effects of Loper Bright, but one thing is already clear: the balance between expertise and interpretation is shifting. The era of automatic deference may be over, but the work of deliberation has only just begun.”
Image from Supreme Court of The United States

Reflecting on the 33rd Animal Law Conference
By Daria Bednarczyk, Blair Eagleson, CriShaun Hardy, Akosua Dufie, Matthew Durthaler, Zachary Liebowitz, and Rebecca Critser | October 30th, 2025
“...our team hopes to strengthen our shared mission and continue growing as individual professionals dedicated to ethical, evidence-based progress.”
Image provided by Shannon Dixon

AOPs, Space, and In-Silico
By Awurakua Asamoah-Mensah | October 16th, 2025
"AOP development is pivotal in guiding regulatory decisions, bridging knowledge gaps, and predicting stimulus impact. Further integrating new approach methodologies, such as in-silico models, into research will enhance AOP development, offering novel and relevant contributions to science, medicine, and policy."
Image by WikiImages

World Congress on Alternative 13: A Critical Incubator for the Development of New Approach Methodologies
By Rebecca Critser | October 2nd, 2025
“[Policy and law] conversations will become especially important as the field moves rapidly out of the research and development phase and into the implementation phase.”
Image by Rebecca Critser

Professional Perspectives: A New Federal Bill Would Bring Government-Funded Animal Experiments Out of the Shadows
By Sadie Jacobs | September 18th, 2025
“The public has a right to know how taxpayer money is being used—especially when it involves sentient beings enduring pain and distress in government-funded experiments. The FARA Act provides a practical, bipartisan solution to a long-standing problem: the lack of transparency in federal animal experiments.”
Image by Paul Locke
Transitioning to Human-Centered Science: An Off-Ramp and Transition Plan
By Paul Locke | September 4th, 2025
“For those of us who have been advocating for the development, use and adoption of human centered NAMs, the Roadmap and these announcements are a breath of fresh air. It seems that – finally – the federal government has turned the corner. But talking the talk is not enough. The transition to human centered science and away from animal testing needs an implementation plan …”
Image by FDA

Professional Perspectives: Wisconsin Court Orders Criminal Investigation of Beagle Breeder Ridglan Farms
By Conley Wouters | August 21st, 2025
“Regardless of the outcome of the investigation, Judge Lanford’s opinion highlights evidence of decades of egregious cruelty and immense animal suffering at Ridglan Farms. That alone might catalyze a renewed push for change from those who are working to protect—and eventually replace—animals used in research.”
Image by azerbaijan_stockers

From Option to Obligation: The Legal Imperative for NAMs
By Jo Anderson | August 7th, 2025
“Regulatory agencies must move from encouraging NAMs to requiring them where scientifically justified. Once merely regarded as alternatives to animal testing, NAMs have now emerged as a driving force in regulatory science.”
Image by NIH
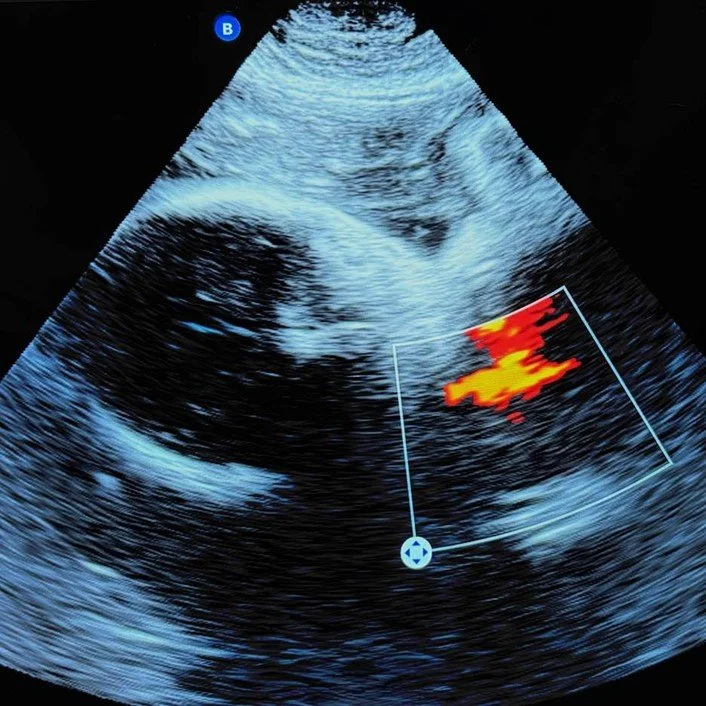
A Bright Tomorrow for Therapeutic Orphans: The Potential for New Approach Methodologies to Bridge Data Gaps for Pregnant Patients
By Matthew Durthaler | July 24th, 2025
“As caution often precludes pregnant humans from serving as models of their own physiology, various non-human animal models have been employed. While these models have displayed some value, they are severely limited by interspecies variation and thus serve as subpar predictors of gravid human physiology.”
Sonogram captured by Matthew Durthaler, used with permission

Our Tiny Allies in Toxicological Testing Part 2 – Transparent Roundworms as Alternative Models
By Aaradhya Diwan | July 10th, 2025
“There are numerous opportunities for using C. elegans as a model for toxicity testing, thanks to their unique advantages and translational relevance to human health, as demonstrated in applications to neuroscience and developmental biology.”
Image by Zeynep F. Altun

Unveiling IACUC members: Triumph for rule of law and animal welfare or a dangerous precedent? A closer look at P. Poe 5 et al. v University of Washington et al.
By Akosua Dufie and Jo Anderson | June 26th, 2025
“While privacy and transparency appear to be contradictory in this instance, their practical implementation are not mutually exclusive. With a careful balancing strategy, both needs can be accommodated and satisfied.”
Image by Pexels

The Silent Strain: Compassion Fatigue in Animal Research Labs
By Shannon Dixon | June 12th, 2025
“Compassion fatigue is not a flaw or a weakness. It is a human response and a natural consequence of empathy.”
Image by Pexels

NAMs and the Funding Frontier: How Modern Toxicology Maximizes Scientific Return on Investment
By Zachary Liebowitz | May 29th, 2025
“Funders are not only looking for scientific merit—they are also scrutinizing value and feasibility. NAMs answer all three.”
Image by ChatGPT

From Roadmap to Reality: Validating NAMs for FDA's Plan to Phase Out Animal Testing
By Yiguang Zhu | May 15th, 2025
“The FDA and the broader scientific community recognize NAMs as a means to obtain 'faster and more accurate human risk assessments' while reducing animal use.”
Image by ChatGPT

Professional Perspectives: Guest Blog by Madeline Krasno, Justify Executive Director
By Madeline Krasno | May 1st, 2025
“What does it reveal about an industry that prides itself on rigorous oversight, ethical review processes, and mental health resources when its own workers feel compelled to voice concerns only under total anonymity?”
Image by Justify

Advancing NAMs for Risk Assessment: Perspectives from SOT 2025
By Breanne Kincaid | April 17th, 2025
“Together, these presentations illustrate a clear shift towards integrated, mechanistically informed risk assessment strategies that reduce the number of animals required to generate toxicity data—paving the way for regulatory frameworks that are both scientifically robust and economically sustainable.”
Image by Breanne Kincaid